Sucralose CAS 56038-13-2 Assay 98.0~102.0% Factory
Ruifu Chemical is the leading manufacturer of Sucralose (CAS: 56038-13-2) with high quality, production capacity 8000 tons per year, can meet the USP/EP/FCC specifications. Sucralose is used as a food additive artificial sweetener or pharmaceutical excipient. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Sucralose, Please contact: alvin@ruifuchem.com
| Chemical Name | Sucralose |
| Synonyms | 1-(1,6-Dichloro-1,6-Dideoxy-β-D-Fructofuranosyl)-4-Chloro-4-Deoxy-α-D-Galactopyranoside; 1,6-Dichloro-1,6-Dideoxy-beta-D-Fructofuranosyl-4-Chloro-4-Deoxy-alpha-D-Galactopyranoside; E955; Trichlorosucrose; 1',4,6'-Trichlorogalactosucrose; Splenda |
| Stock Status | In Stock, Production Capacity 8000 Tons per Year |
| CAS Number | 56038-13-2 |
| Molecular Formula | C12H19Cl3O8 |
| Molecular Weight | 397.63 g/mol |
| Melting Point | 130℃ |
| Density | 1.375 g/cm3 |
| Sweetness | 600 x Sucrose |
| Water Solubility | Soluble in Water |
| Stability | Hygroscopic |
| COA & MSDS | Available |
| Shelf Life | 24 Months When Properly Stored |
| Origin of Product | China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystalline Powder | White Crystalline Powder |
| Identification | A: IR, B: HPLC | Complies |
| Specific Rotation at 20℃ | +84.0° ~ +87.5° (C=1 in H2O) | +86.4° |
| pH of 10% Aqucous Solution | 5.0~8.0 | 6.3 |
| Water Determination | <2.00% | 0.11% |
| Residue on Ignition | <0.70% | <0.70% |
| Limit of Methanol | <0.10% | Not Find |
| Heavy Metals (Pb) | <10ppm | <10ppm |
| Arsenic (As) | <3ppm | <3ppm |
| Lead (Pb) | <1ppm | <1ppm |
| Iron (Fe) | <10ppm | <10ppm |
| Limit of Hydrolysis Products | <0.10% (Chlorinated Monosaccharides) | <0.10% |
| Related Substances | <0.50% (Other Chlorinated Disaccharides) | <0.50% |
| Assay / Analysis Method | 98.0~102.0% (on the Anhydrous Basis) | 99.73% |
| Microbiological Analysis | ||
| Total Plate Count | <250cfu/g | <250cfu/g |
| Yeast & Mold | <50 cfu/g | <50cfu/g |
| E. Coil | Negative | Negative |
| Coliform | Negative | Negative |
| Salmonella / 25g | Negative | Negative |
| Staphylococcus Aureusa / 25g | Negative | Negative |
| Conclusion | Has been tested and complies with the USP/EP/FCC specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (<21℃), well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.
Sucralose
C12H19Cl3O8 397.64
1,6-Dichloro-1,6-dideoxy-d-fructofuranosyl-4-chloro-4-deoxy-d-galactopyranoside;
1,4,6-Trichlorogalactosucrose [56038-13-2].
DEFINITION
Sucralose contains NLT 98.0% and NMT 102.0% of C12H19Cl3O8, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption <197K>
• B. The retention time of the principal peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. The RF value of the principal spot of the Sample solution corresponds to that of Standard solution A, as obtained in the test for Related Compounds.
ASSAY
• Procedure
Mobile phase: Acetonitrile and water (3:17)
Standard solution: 1 mg/mL of USP Sucralose RS in Mobile phase
Sample solution: 1 mg/mL of Sucralose in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: Refractive index
Column: 8-mm × 10-cm; packing L1
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
[Note-The retention time of sucralose is about 9 min.]
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of sucralose (C12H19Cl3O8) in the portion of Sucralose taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of the Sample solution
rS = peak response of the Standard solution
CS = concentration of USP Sucralose RS in the Standard solution (mg/mL)
CU = concentration of Sucralose in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition <281>: NMT 0.7%
• Heavy Metals, Method II <231>: 10 ppm
• Limit of Methanol
Internal standard solution: 0.1 µL/mL of n-propyl alcohol in pyridine
Standard solution: 0.2 µL/mL of methanol in Internal standard solution
Sample solution: 0.2 g/mL of Sucralose in Internal standard solution
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 4-mm × 2-m glass column; packed with 80- to 100-mesh silanized support S6
Temperature
Column: 150
Detector: 250
Injector: 200
Carrier gas: Helium
Flow rate: 20 mL/min
Injection size: 1 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of methanol in the portion of Sucralose taken:
Result = (RU/RS) × [(CS/CU) × F1] × F2 × 100
RU = peak response ratio of methanol to n-propyl alcohol, from the Sample solution
RS = peak response ratio of methanol to n-propyl alcohol, from the Standard solution
CS = concentration of methanol in the Standard solution (µL/mL)
CU = concentration of Sucralose in the Sample solution (g/mL)
F1 = conversion factor from µL to mL
F2 = specific gravity of methanol, 0.79 g/cm3
Acceptance criteria: NMT 0.1%
• Related Compounds
Adsorbent: 0.20-mm layer of octadecylsilanized chromatographic silica gel. The thin-layer chromatographic plate also has a preadsorbent zone.
Detection reagent: Sulfuric acid in methanol (3 in 20)
Standard solution A: 10.0 mg/mL of USP Sucralose RS in methanol
Standard solution B: 0.5 mL Standard solution A diluted to 10.0 mL with methanol
Sample solution: 100.0 mg/mL of Sucralose in methanol
Developing solvent system: Acetonitrile and sodium chloride solution (1 in 20) (3:7)
Application volume: 5 µL
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Proceed as directed under Chromatography <621>, Thin-Layer Chromatography. Spray the plate with Detection reagent. Heat the plate for 10 min at 125.
Acceptance criteria: The RF value of the principal spot from the Sample solution corresponds to that obtained from Standard solution A, and the color of any other single spot from the Sample solution is not more intense than that of the principal spot from Standard solution B (0.5%).
• Limit of Hydrolysis Products
[Note-This test does not require a developing solvent.]
Adsorbent: 0.25-mm layer of chromatographic silica gel
Spray reagent: 12.3 mg/mL of p-anisidine and 16.6 mg/mL of phthalic acid in methanol. Store the solution in the dark and refrigerate to prevent discoloration. Discard if the solution becomes discolored. [Caution—p-Anisidine is toxic if inhaled or if absorbed through the skin.]
Standard solution A: 100 mg/mL of mannitol
Standard solution B: 0.4 mg/mL of fructose and 100 mg/mL of mannitol
Sample solution: 250 mg/mL of Sucralose in methanol
Application volume: 5-µL portions separately applied in 1-µL increments, allowing the plate to dry between applications
Analysis
Samples: Standard solution and Sample solution
Proceed as directed under Chromatography <621>, Thin-Layer Chromatography. Spray the plate with Spray reagent, and heat the plate at 100 ± 2 for 15 min. If the spot from Standard solution A has darkened, repeat the test, heating for a shorter period of time. Immediately after heating, view the plate against a dark background.
Acceptance criteria: The color of the spot from the Sample solution is not more intense than that from Standard solution B (0.1%).
SPECIFIC TESTS
• Optical Rotation, Specific Rotation <781S>: +84.0 to +87.5 at 20℃
Sample solution: 10 mg/mL of Sucralose
• Water Determination, Method I <921>: NMT 2.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers, in a cool, dry place, at a temperature not exceeding 21℃.
• USP Reference Standards <11>
USP Sucralose RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39 - Wear suitable gloves and eye/face protection
S24/25 - Avoid contact with skin and eyes.
WGK Germany 3
RTECS LW5440140
HS Code 2932140000
Toxicity LD50 orally in Rabbit: > 10000 mg/kg
Sucralose (CAS: 56038-13-2) is an artificial sweetener that is used as a sugar substitute in a wide variety of foods and beverages. It is a non-caloric, non-nutritive sweetener that is 600 times sweeter than regular sugar. It has been approved for use in over 80 countries, and is one of the most widely used artificial sweeteners in the world. It is also known as Splenda, a brand name owned by McNeil Nutritionals.
Sucralose is a high-strength sweetener with a molecular formula of C12H19Cl3O8. High stability, stable to light, heat and PH. It is very soluble in water, methanol and ethanol, and slightly soluble in ether.
Sucralose is an artificial sweetener that is commonly used in various food and beverage products. It was discovered by researchers at Tate & Lyle and initially introduced to the market in the 1990s under the brand name Splenda. Sucralose is derived from sucrose, which is a natural sugar present in plants and fruits. Unlike sucrose, which is a calorie-containing sugar, sucralose is a zero-calorie sweetener that is commonly used as a sugar replacement.
1. Sucralose as sweetness is 600-650 times than sugar;
2. Sucralose with good solubility and stability, is pure taste,has no odor;
3. Sucralose without any safety toxicological aspects of doubt,energy dissipations is 0, won't cause fat;
4. Sucralose's available for diabetes cerebrovascular disease patients and old people to use.Sucralose will not cause caries and the teeth healthy.
1) Carbonated drinks and still beverages
2) Jams, jelly, milk products, syrup, confections
3) Baked goods, desserts
4) Ice cream, cake, pudding, wine, fruit can, etc
Sucralose (CAS: 56038-13-2) has potential implications in various fields of research and industry. In food and beverage, sucralose can be used as a sugar substitute to reduce calorie intake without compromising taste. The use of sucralose can also help reduce the risk of dental caries. In the pharmaceutical industry, sucralose can be used as a coating for drugs to mask unpleasant tastes. In biotechnology, sucralose can be used as a carbon source for the fermentation of microorganisms.
The FDA, in April 1998, approved Sucralose (CAS: 56038-13-2) for use as a tabletop sweetener and as an additive in a variety of food products. In the UK, Sucralose was fully authorized for use in food products in 2005. It is also accepted for use in many other countries worldwide. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
-
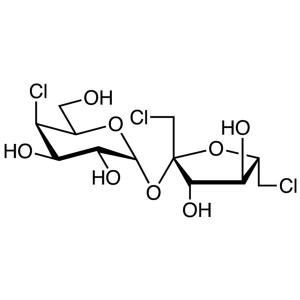
Sucralose CAS 56038-13-2 Assay 98.0~102.0% Factory
-
D-Glucuronolactone CAS 32449-92-6 Assay 98.5%~1...
-
meso-Erythritol CAS 149-32-6 Assay 99.5~100.5% ...
-
D-(+)-Xylose CAS 58-86-6 Purity >99.5% (HPLC) F...
-
D-(+)-Raffinose Pentahydrate CAS 17629-30-0 Ass...
-
D-(+)-Trehalose Dihydrate CAS 6138-23-4 Assay >...
-
D-(+)-Mannose CAS 3458-28-4 Assay >99.0% (HPLC)...
-
D-Tagatose CAS 87-81-0 Assay >99.0% (HPLC) Factory
-
D-Glucal CAS 13265-84-4 Assay >96.0% (HPLC) Fac...
-
D-(+)-Glucose Anhydrous CAS 50-99-7 Assay ≥99.5...
-
D-Mannosamine Hydrochloride CAS 5505-63-5 Assay...
-
D-(+)-Galactose Anhydrous CAS 59-23-4 Assay >98...
-
D-(+)-Glucosamine Hydrochloride CAS 66-84-2 Ass...
-
D-(+)-Maltose Monohydrate CAS 6363-53-7 Assay ≥...
-
Lactulose CAS 4618-18-2 Assay >98.5% (HPLC)
-
DL-Dithiothreitol (DTT) CAS 3483-12-3 Assay >98...

