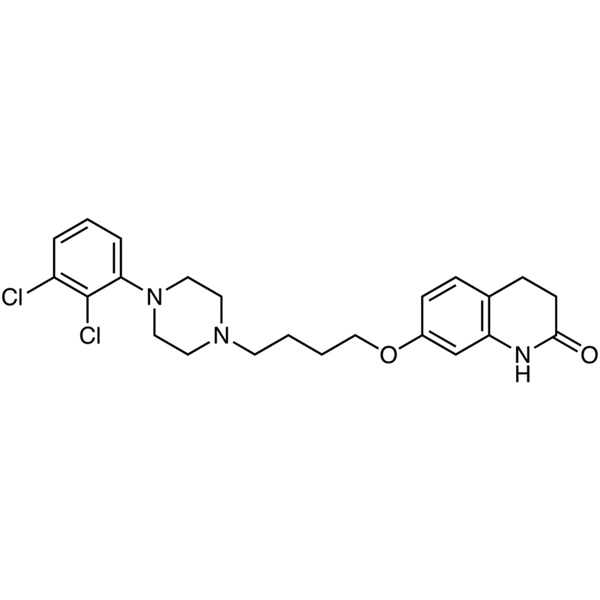
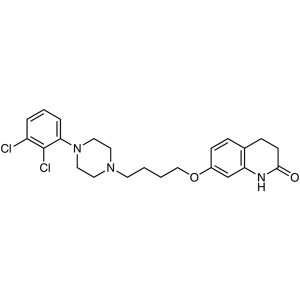
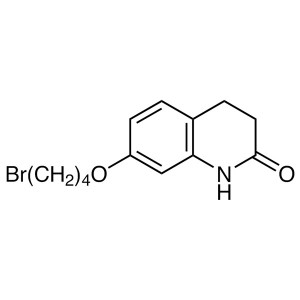
Aripiprazole CAS 129722-12-9 Assay 98.0%~102.0% API High Quality
Ruifu Chemical is the leading supplier of Aripiprazole (CAS: 129722-12-9) with high quality, API, antipsychotic.
Purchase Aripiprazole or its intermediates, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Aripiprazole |
| Synonyms | 7-[4-[4-(2,3-Dichlorophenyl)-1-Piperazinyl]butoxy]-3,4-Dihydro-2(1H)-Quinolinone |
| CAS Number | 129722-12-9 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C23H27Cl2N3O2 |
| Molecular Weight | 448.39 |
| Melting Point | 136.0~140.0℃ |
| Density | 1.263±0.06 g/cm3 |
| Solubility | Insoluble Soluble in Water; Insoluble in Methanol |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Almost White Crystalline Powder |
| Infrared Spectroscopy | Conforms to the Structure |
| Solubility | Soluble in Acetic Acid, Slightly Soluble in Ethanol |
| Melting Point | 136.0~140.0℃ |
| Loss on Drying | ≤0.50% (105℃ for 3 h) |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤20ppm |
| Related Substances | |
| Aripiprazole Related Compound G | ≤0.10% |
| Aripiprazole Related Compound F | ≤0.10% |
| Aripiprazole 4,4′-Dimer | ≤0.10% |
| Any Other Individual Impurity | ≤0.10% |
| Total Impurities | ≤0.50% |
| Assay / Analysis Method | 98.0%~102.0% (Calculated on the Dried Basis) |
| Micro Limits | |
| Total Aerobic Microbial Count | ≤1000 cfu/g |
| Yeast and Mold Combined | ≤100 cfu/g |
| E.Coli | Absence |
| Pathogenic Organizing | Absence |
| Test Standard | Enterprise Standard |
Package: Bottle, 5kg/aluminum tin, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry and well-ventilated warehouse away from incompatible substances. Keep away from direct sunlight; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.

Risk Codes
R11 - Highly Flammable
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
R36 - Irritating to the eyes
Safety Description
S16 - Keep away from sources of ignition.
S36/37 - Wear suitable protective clothing and gloves.
UN IDs UN 1993C 3 / PGIII
WGK Germany 3
RTECS VC8275950
HS Code 2934 9990.91
Aripiprazole (CAS: 129722-12-9) is a new kind of highly lipid soluble quinoline derivatives, its pharmacological effects characteristic is that it is not only the postsynaptic dopamine D2 receptor antagonist, but also the presynaptic dopamine D2 receptor agonist,it can also excite D1, D3, D4 receptors. Aripiprazole is a second generation atypical antipsychotic and anti-depressant with partial agonist activity at dopamine D2 and serotonin 5-HT1A receptors and antagonist activity at serotonin 5-HT2A receptors. Ki values are 0.34 nM, 0.8 nM, 1.7 nM, and 3.4 nM, respectively, for dopamine D2 and D3, serotonin 5-HT1A and 5-HT2A receptors. Aripiprazole is used for the treatment of schizophrenia and related psychotic disorders. Bristol-Myers Squibb and Otsuka Pharmaceutical Company announced that the European Union has approved Abilify (Aripiprazole) in the treatment of schizophrenia listing application. Schizophrenia affects 1% of the global population, and more in young adults. Schizophrenia affects thinking, emotional control and decision-making ability of the patient. Schizophrenia-positive patients will have symptoms such as hallucinations and delusions, patients with negative symptoms are social withdrawal, lack of emotional changes. In 2002 the FDA approved Abilify for the treatment of schizophrenia, which has five dosage strengths: 5 mg, 10 mg, 15 mg, 20 mg and 30 mg, since its approval.
Aripiprazole
C23H27Cl2N3O2 448.39
2(1H)-Quinolinone, 7-[4-[4-(2,3-dichlorophenyl)-1- piperazinyl]butoxy]-3,4-dihydro-;
7-[4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril [129722-12-9]; UNII: 82VFR53I78.
DEFINITION
Aripiprazole contains NLT 98.0% and NMT 102.0% of aripiprazole (C23H27Cl2N3O2), calculated on the dried basis.
IDENTIFICATION
Change to read:
• A. SPECTROSCOPIC IDENTIFICATION TESTS <197>, Infrared Spectroscopy: 197K (CN 1-MAY-2020)
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• PROCEDURE
Protect the solutions from light.
Diluent: Acetonitrile, methanol, water, and acetic acid (30:10:60:1)
Solution A: Acetonitrile and 0.05% triuoroacetic acid (10:90)
Solution B: Acetonitrile and 0.05% triuoroacetic acid (90:10)
Mobile phase: See Table 1.
Table 1
Time (min) Solution A (%) Solution B (%)
0 80 20
2 80 20
10 65 35
20 10 90
25 10 90
26 80 20
35 80 20
[ NOTE- The gradient was established on an HPLC system with a dwell volume of approximately 650 µL.]
System suitability solution: 1 µg/mL each of USP Aripiprazole RS and USP Aripiprazole Related Compound F RS in Diluent
Standard solution: 0.1 mg/mL of USP Aripiprazole RS in Diluent
Sample solution: 0.1 mg/mL of Aripiprazole in Diluent
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 10-cm; 3-µm packing L1
Flow rate: 1.2 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[ NOTE- The relative retention times for aripiprazole and aripiprazole related compound F are 1.0 and 1.1, respectively.]
Suitability requirements
Resolution: NLT 2.0 between aripiprazole and aripiprazole related compound F, System suitability solution
Tailing factor: NMT 1.5 for aripiprazole, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of aripiprazole (C23H27Cl2N3O2) in the portion of Aripiprazole taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak area from the Sample solution
rs = peak area from the Standard solution
Cs = concentration of USP Aripiprazole RS in the Standard solution (mg/mL)
Cu = concentration of Aripiprazole in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
IMPURITIES
• RESIDUE ON IGNITION <281>: NMT 0.1%
• ORGANIC IMPURITIES
Protect the solutions from light.
Diluent, Solution A, Solution B, Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic
system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Aripiprazole taken:
Result = (ri /ru ) × (1/F) × 100
ri = peak response of each impurity from the Sample solution
ru = peak response of Aripiprazole from the Sample solution
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
Table 2
Name Relative Retention Time Relative Response Factor Acceptance Criteria, NMT (%)
Aripiprazole related compound G a 0.9 0.72 0.10
Aripiprazole 1.0- -
Aripiprazole related compound F bc 1.1 1.0 0.10
Aripiprazole 4,4′-dimer d 1.3 1.0 0.10
Any other individual impurity - 1.0 0.10
Total impurities - - 0.50
a 7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}quinolin-2(1H)-one.
b 4-(2,3-Dichlorophenyl)-1-[4-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yloxy)butyl]piperazine 1-oxide.
c If possible from the manufacturing process.
d 1,1′-(Ethane-1,1-diyl)bis(2,3-dichloro-4-{4-[3,4-dihydroquinolin-2(1H)-one-7-yloxybutyl]piperazin-1-yl}benzene).
SPECIFIC TESTS
• LOSS ON DRYING <731>
Analysis: Dry at 105℃ for 3 h.
Acceptance criteria: NMT 0.5%
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in tight containers. Store at controlled room temperature.
• USP REFERENCE STANDARDS <11>
USP Aripiprazole RS
USP Aripiprazole Related Compound F RS
4-(2,3-Dichlorophenyl)-1-[4-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yloxy)butyl]piperazine 1-oxide.
C23H27Cl2N3O3 464.38